Goals
- Development and validation of new medical image reconstruction methods based on compressive sensing, focusing on real-world implementation of computed tomography (CT) and magnetic resonance (MR) imaging.
- Creation of a testbed of common classes of medical image processing algorithms (denoising, deblurring, registration, segmentation) exhibiting different parallelism motifs to motivate CDSC research.
- Demonstration of new image processing methods with improved performance based on acceleration via the customizable heterogeneous platform (CHP).
People
- Thrust Leader: Alex Bui (UCLA)
- Faculty:
- UCLA: Denise Aberle, Aichi Chien, William Hsu, Kyung Sung, Luminita Vese
- Rice: Rich Baraniuk
- Graduate students & Postdocs:
- UCLA: Egil Bae, Norin Duggan, Shiwen Shen, Ming Yan
- Rice/UCLA: Jianing Shi
- UCSB: Xin Yang
Focused Areas:
Image Reconstruction Algorithms, Medical Image Processing Pipeline
Image Reconstruction Algorithms
In 2006, seminal work led to the development of compressed sensing theory, which proved that a signal can be reconstructed perfectly from a limited number of samples (i.e., significantly less than the Nyquist rate) if the given signal is sparse in some transform domain. For medical imaging, compressed sensing implies that the number of samples used to reconstruct an imaging study can be decreased – without sacrificing image accuracy – if the target image is already sparse (e.g., an angiogram) or a sparsifying transform can be determined. However, the inherent challenge in applying compressive sensing to medical imaging is that: 1) no single technique has thus far been found to be optimal; and 2) the underlying computational complexity of the algorithms, making them untenable for real-world clinical usage.
CT reconstruction. Advances in computed tomography (CT) scanner hardware permit high resolution anatomical and functional information to be extracted. However, cumulative radiation exposure has been a growing area of concern within the medical imaging community as the use of CT continues to increase. For example, clinicians weigh the risk associated with routine chest CT screening for lung cancer and identifying patients at high risk of lung cancer at an early stage. An ongoing debate within the medical community is whether routine lung cancer screening is beneficial given the risks associated with radiation exposure. These concerns have spurred development of low-dose acquisition protocols. While the reduction in current results in reduced radiation exposure, acquired images have poorer signal-to-noise ratio. Other approaches include utilizing iterative reconstruction techniques rather than traditional filtered back projection (FBP). Markedly, thoracic imaging remains a particularly difficult area given the need to resolve fine details of the lung anatomy (e.g., bronchioles) to identify potential malignant nodules or characterize areas that may be an early indicator of disease. For CT, the key result from compressive sensing is that the number of required projections can be reduced while still preserving image quality. Importantly, angular undersampling can potentially be used to lessen the overall amount of radiation used during study acquisition. To that end, we have developed the EM+TV algorithm (Expectation-Maximization and Total Variation). EM+TV is centered on solving the following, where x represents the image, b the observations with noise (i.e., the measurements from the scanner), and A is a transform:
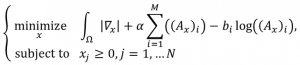
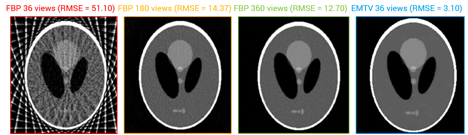
EM is an iterative optimization step, attempts to maximize the probability of the observations with noise (b) given the original image (x) and a transform (A); while also minimizing the total variation. TV provides image regularization. A naïve implementation of EM+TV required ~18 hours to complete on a standard sized CT dataset (e.g., 512 x 512 image, 128 slices). Through the collaborative efforts of CDSC, this algorithm can now be run in ~6 minutes, which make this technique a viable replacement for FBP. Results for a Shepp-Logan phantom are shown below. CDSC has compared the visual quality and diagnostic impact of EM+TV on real-world low-dose CT patient studies, comparing current iterative and FBP reconstructions against subsampled CT studies. Image quality has been evaluated by utilizing the appropriate quantitative metrics such as standard root mean square error (RSME), universal quality index (UQI), and correlation coefficient (CC). Compared to traditional FBP, EM+TV yields significantly better results in all three metrics when reconstructing the low-dose images.
- [FTDIRRNM11C] Chen J, Yan M, Vese LA, Villasenor J, Bui AAT, Cong J. EM+TV for reconstruction of cone-beam CT with curved detectors using GPU. Proc 11th Intl 3D Image Reconstruction in Radiology and Nuclear Medicine, July 2011.
- [LNCS11Y] Yan M. EM-Type algorithms for image reconstruction with background emission and Poisson noise. Advances in Visual Computing; Lecture Notes in Computer Science, 2011, Volume 6938/2011, 33-42.
- [SPIE11Y] Yan M, Vese LA. Expectation maximization and total variation-based model for computed tomography reconstruction from undersampled data. In Proceedings SPIE, volume 7961, 2011.
- [ISVC11Y] Yan M, Chen J, Vese LA, Villasenor J, Bui AAT, Cong J. EM+TV based reconstruction for cone-beam CT with reduced radiation. Proc Intl Symp Visual Computing, September 2011:1-10.
- [IPIJ13Y] Yan M, Bui AAT, Cong J, Vese L. General convergent expectation maximization (EM)-type algorithms for image reconstruction. Inverse Problems and Imaging Journal, 2013; 7(3):1007-1029.
- [JCM13Y] Yan M. Convergence analysis of SART: Optimization and statistics. Intl J Computer Mathematics 2013; 90:30-47.
- [ISMRM15S] Sung KY, Wu D, Han F, Zhou Z, Hu P, Wu H, Bui AAT, Cong J. Customized CPU accelerated CS-based MRI reconstruction platform. Proc Intl Soc Magnetic Resonance in Medicine (ISMRM), May 2015.
- IEEE James H. Mulligan, Jr. Education Medal “for fundamental contributions to open educational resources for electrical engineering and beyond”.
- IEEE Signal Processing Society Technical Achievement Award.
- Thomson Reuters’ Highly Cited Researcher.
- Presidential Mentoring Award (Rice)
MR reconstruction. For MR, compressive sensing has the potential to both improve image quality while speeding acquisition; significant research is being conducted in this area. CDSC’s Application Thrust has been developing new methods for dynamic MR image reconstruction. This work, led by Rice University collaborators, has involved: 1) devising novel models for video compressive sensing and developing numerical algorithms that are suitable for parallel computing architecture; 2) applying video compressive sensing techniques to accelerate the acquisition process of dynamic MRI and demonstrating its success through quality of reconstruction and compression rate; and 3) accelerating the computational speed of these numerical algorithms through the state-of-the-art parallel computing architectures. Two novel models for video compressive sensing have been developed:
- One model utilizes a linear dynamical systems model and is called CS-LDS. The CS-LDS model was extended to the Fourier domain and applied it to accelerate the acquisition process of dynamic MRI. The CS-LDS algorithm was accelerated by translating the original Matlab code to the CnC language, achieving a four-fold speedup on a four-core computer
- The other model is based on multi-scale video recovery and is termed CS-MUVI. Given highly under-sampled data in the k-t space, we first estimate a low-resolution version of the video at a sequence of sub-sampled time points based on a customized sensing matrix in the Fourier domain. The spatial and temporal resolution of this video is optimized for the best image quality, given the time varying nature of dynamic imaging and the uncertainty principle. Optical flow is consequently estimated between consecutive frames of this reconstructed low-resolution video.
- [CNS12S] Shi JV, Sankaranarayanan AC, Studer C, Baraniuk RG. Video compressive sensing for dynamic MRI. Proc Annual Computational Neuroscience Meeting (CNS), Atlanta, GA, July 21-26, 2012.
- [ICCP12S] Sankaranarayanan C, Studer C, Baraniuk RG. CS-MUVI: Video compressive sensing for spatial-multiplexing cameras. Proc IEEE Intl Conf Computational Photography, Seattle, WA, April, 2012.
- [TIP12D] Duarte MF, Baraniuk RG. Kronecker compressive sensing. IEEE Trans Image Processing, 21(2):494-504, 2012.
- [CnC12B] Baraniuk RG, Budimlic Z, Burke M, Imam S, Knobe K, Sarkar V, Shi JV. Parallelizing compressive sensing MRI via CnC-Babel and Matlab. 4th Annual Concurrent Collections (CnC) Workshop, 2012.
- [SAHD13S] Shi V, Yin W, Sankaranarayanan AC, Baraniuk RG. Video compressive sensing for dynamic MRI. Duke Workshop on Sensing and Analysis of High-Dimensional Data (SAHD), 2013.
- [ISMRM15S] Sung KY, Wu D, Han F, Zhou Z, Hu P, Wu H, Bui AAT, Cong J. Customized CPU accelerated CS-based MRI reconstruction platform. Proc Intl Soc Magnetic Resonance in Medicine (ISMRM), May 2015.
Medical Image Processing Pipeline
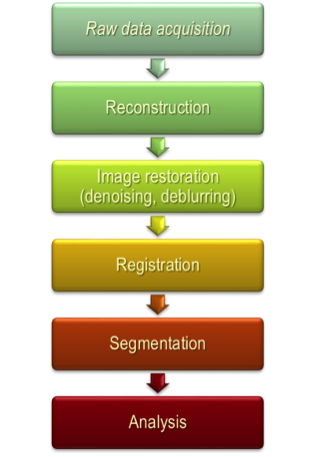
A focus of the Application Thrust has been in the design of a suite of medical image processing algorithms to motivate the software and hardware developments in the other CDSC Thrusts. To further consolidate the problem space, we have implemented a pipeline of algorithms, following frequently employed processing steps used in medical image analysis. Dependent on the application domain and need, different algorithms are substituted at each step. This modularity has provided the basis for design of the CHP (as different algorithms present differently and exhibit different runtime behaviors), as well as compiler developments as code was translated from Matlab through to different implementations (C/C++; CUDA; RTL; etc.). An overarching application for this pipeline is in the automatic detection and segmentation of a tumor in serial chest CT images, providing a means to compute the volume of the tumor as the patient receives treatment (thereby indicating response to a given intervention).
- Code release: http://code.google.com/p/cdsc-image-processing-pipeline/.
- [LNCS11G] Getreuer P, Tong M, Vese LA. A variational model for the restoration of MR images corrupted by blur and Rician noise. Advances in Visual Computing, Lecture Notes in Computer Science, 2011; 6938:686-698.
- [ASPDAC12B] Bui AAT, Cheng KT, Cong J, Vese L, Wang YC, Yuan B, Zou Y. Platform characterization for domain-specific computing. Proc 17th Asia and South Pacific Design Automation Conference (ASP-DAC) 2012:94-99
- [CSE11H] Hsu W, Vese L, Bui AAT. Accelerating medical image processing using domain-specific computing. Mini Symposium, 2011 Society for Industrial and Applied Mathematics (SIAM) Conference on Computational Science and Engineering, Feb. 2011; Reno, Nevada.
- [Radiology11Y] Yan M, Zou Y, Hsu W, Chien A, Vese L, Aberle DR, Bui AAT, Cong J. Accelerating image reconstruction and analysis using domain-specific computing. RSNA Annual Meeting, Chicago, IL; Nov 2011, Radiology(P):312.
- [JIS14Y] Yan M. Restoration of images corrupted by impulse noise and mixed Gaussian impulse noise using blind inpainting, SIAM Journal on Imaging Sciences, 2014.
- [JSC14Y] Yan M, Yang Y, Osher S. Exact low-rank matrix completion from sparsely corrupted entries via adaptive outlier pursuit. Journal of Scientific Computing, 2014.
- [LNCS11L] Lederman C, Vese L, Chien A. Registration for 3D morphological comparison of brain aneurysm growth. Advances in Visual Computing, Lecture Notes in Computer Science, 2011; 6938:392-399.
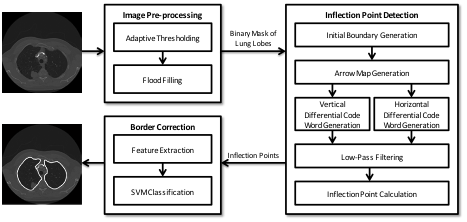
Lung nodule detection. With increasing attention to the importance of lung cancer screening to monitor and respond early to potential disease, there has been a call for new methods that can assist in the automatic detection of suspicious nodules from low-dose CT studies. A longstanding issue for lung computer-aided detection (CAD) systems is that most nodules attached to the lung wall are very hard to detect: often, these nodules are considered as part of the chest wall and are removed from the region of interest at the initial step by the CAD system. A novel automatic lung segmentation using bidirectional chain codes approach has been developed for improving juxtapleural nodule detection accuracy while minimizing the segmentation error. The method mainly includes four parts: 1) image preprocessing; 2) inflection point detection; 3) feature extraction; and 4) border correction. The method were evaluated on 233 CT studies containing at total of 403 juxtapleural nodules. 92.6% of the nodules are re-included using this method. Segmentation accuracy was further validated on 10 randomly selected CT series, finding a 0.3% average over-segmentation ratio and 2.4% under-segmentation rate when compared to manually segmented reference standards done by an expert. Following figures show the basic steps and their output for the developed border correction method. Combined with the border correction approach, the basic steps for the whole CAD pipeline are detailed as follows:
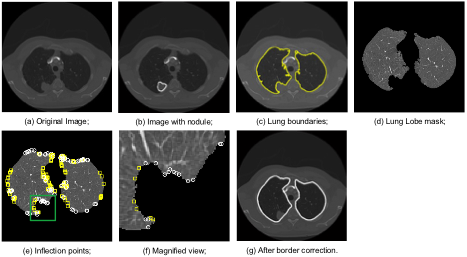
- A two-phase active contour segmentation algorithm is applied to generate initial segmentation results.
- Lung lobe regions are then recognized and a border correction method is employed to modify the lobe boundary to overcome under-segmentation for the region of real nodules attached to lung wall.
- A set of rules are defined to detect nodule candidates, which are combined with a nodule/vessel separation method.
- 44 texture and morphological features are extracted for all remaining nodule candidates. A genetic algorithm is used to select the optimal combination of features for the classifier.
- Finally, a k-means classifier is used to differentiate lung nodules from other objects using the optimal features.
This lung nodule detection method has been validated against the and EMTV reconstructed low-does data and Lung Imaging Database Consortium (LIDC) dataset, which provide gold standard annotation markup for nodules across a large dataset of thoracic CT imaging studies. Initial segmentation and classifier results indicate that this approach provides a good balance between false positive and negative performance, erring conservatively on the side of false positives. The CAD pipeline is accelerated using stencil compilation in C++ reducing the running time to 2-5 minutes on multicore CPU. The optimized CAD pipeline is integrated with FPGA reconstruction module as an adaptive reconstruction prototype. This prototype is able to reconstruct suspicious image slices based on the nodule detection results.
- [CBM15S] Shen S, Bui AAT, Cong J, Hsu W. An automated lung segmentation approach using bidirectional chain codes to improve nodule detection accuracy. Computers in Biology and Medicine 57 (2015): 139-149.
- [EMMCVPR15D] Duggan N, Bae E, Shen S, Hsu W, Bui AAT, Jones E, Glavin M, Vese L. A technique for lung nodule candidate detection in CT using global minimization methods. In Energy Minimization Methods in Computer Vision and Pattern Recognition, pp. 478-491. Springer International Publishing, 2015.
Vessel segmentation. We have developed techniques and algorithms: 1) to simplify parameter configuration for existing vessel segmentation methods; and 2) to improve the segmentation accuracy in challenging scenarios (e.g., in regions with low contrast and signal-to-noise-ratios (SNR); at vessel boundaries with disturbance induced by adjacent non-vessel pixels). Specifically, we have designed an adaptive configuration method based on shape filtering for Geodesic Active Contour (GAC), one of the most popular methods for vessel segmentation. An evaluation study over clinical cerebral datasets demonstrates that this method achieves greater segmentation accuracy than two popular active contour methods with manually optimized parameters. To improve segmentation performance in low contrast, low SNR regions, we have also developed a progressive contrast enhancement method that adaptively improves the contrast of challenging pixels, suppressing noise by weighting pixels according to their likelihood of being vessel pixels. Experimental results on a public retinal dataset (DRIVE) and our neuroimaging datasets demonstrate that our approach outperforms state-of-the-art methods (e.g., vesselness of Frangi et al.; optimally oriented flux, OOF).
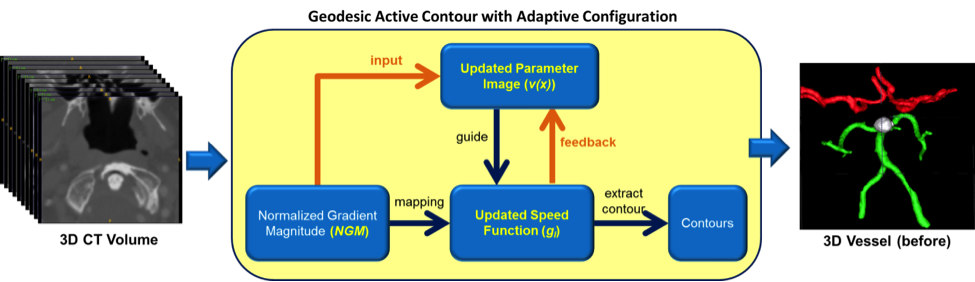
- [ICPR14Y] Yang X, Cheng KT, Chien A. Geodesic active contours with adaptive configuration for cerebral vessel and aneurysm segmentation. Proc Intl Conf Pattern Recognition (ICPR), Stockholm, Sweden, August 24-28, 2014.
- [ACCV15Y] Yang X, Cheng KT, Chien A. Accurate vessel segmentation with progressive contrast enhancement and Canny refinement. InProc Computer Vision (ACCV) 2014, pp. 1-16. Springer International Publishing, 2015.

